| 11234655303 | macromolecule | a large macromolecule formed by the joining of smaller molecules( subunit), usually by a dehydration chemical reaction. | 0 | |
| 11234655338 | Four classes of biological macromolecules | Proteins, carbohydrates, nucleic acids, lipids | 1 | |
| 11234655304 | polymer | a long molecule consisting of many similar or identical monomers linked together by covalent bonds. | 2 | |
| 11234655305 | monomer | the subunit that serves as the building block of a polymer. | 3 | |
| 11234655306 | dehydration synthesis | a chemical reaction in which two molecules become covalently bonded to each other with the removal of a water molecule. |  | 4 |
| 11234655307 | hydrolysis | a chemical reaction that breaks bonds between two molecules by the addition of water; functions in dis-assembly of polymers to monomers. |  | 5 |
| 11234655308 | protein | a macromolecule consisting of one or more polypeptides chains made up amino acids folded and coiled into a specific three-dimensional structure. | 6 | |
| 11234655339 | Functions of proteins | structural support, catalyst, transport, defense, movement, regulation | 7 | |
| 11234655309 | amino acid | an organic molecule possessing both a carboxyl and an amino group. The monomers of polypeptides. There are 20 different forms. Distinguished by side chains. |  | 8 |
| 11234655310 | peptide bond | the linkage between amino acids forming a covalent bond between the carboxyl group on one amino acid and the amino group on another, formed by a dehydration reaction. |  | 9 |
| 11234655340 | denaturation | loss of a proteins normal 3D structure; can possibly be caused by pH and temperature changes which affect the ionic bonds, hydrogen bonds & hydrophilic interactions |  | 10 |
| 11234655311 | enzyme | a macromolecule serving as a catalyst, a chemical agent that increases the rate of a reaction without being consumed by the reaction. most of them are proteins. | 11 | |
| 11234655312 | carbohydrate | a sugar (monosaccharide) or one of its dimers (disaccharides) or polymers (polysaccharides). Primarily C, H and O. | 12 | |
| 11234655341 | What are the functions of carbohydrates | function as energy source & structure support; examples: simple sugars, and complex polysacchrides, structural suppport examples: cell wall in pant cells, chitin in insects. | 13 | |
| 11234655313 | monosaccharide | the simplest carbohydrate, active alone or serving as a monomer for disaccharides and polysaccharides. Also called simple sugars, they have formulas that are generally some multiple of CH2O (1:2:1). |  | 14 |
| 11234655314 | disaccharide | a double sugar, consisting of two monosaccharides joined by a glycosidic linkage formed by a dehydration reaction. |  | 15 |
| 11234655315 | polysaccharide | a polymer of many monosaccharides, formed by dehydration reactions. |  | 16 |
| 11234655316 | starch | a storage polysaccharide in plants, consisting entirely of glucose monomers joined by x glycosidic linkages. Used for energy storage. | 17 | |
| 11234655317 | glycogen | an extensively branched glucose storage polysaccharide found in the liver and muscle of animals; the animal equivalent of starch. | 18 | |
| 11234655318 | cellulose | a structural polysaccharide of plant cell walls, consisting of glucose monomers joined by B glycosidic linkages. A type of plant starch. | 19 | |
| 11234655319 | lipids | any of a group of large biological molecules, including fats, phospholipids, and steroids, that mix poorly, if at all, with water (hydrophobic). No true monomers. | 20 | |
| 11234655342 | What are the three types of lipids? | fats/oils, phospholipids & steroids | 21 | |
| 11234655320 | fat/oil | a lipid consisting of three fatty acids lined to one glycerol molecule; also called a triacylglycerol or triglyceride. Function as energy storage. | 22 | |
| 11234655321 | saturated | a fatty acid in which all carbons in the hydrocarbon tail are connected by single bonds, thus maximizing the number of hydrogen atoms that are attached to the carbon skeleton. |  | 23 |
| 11234655322 | unsaturated | a faty acid that has one or more double bonds betwen carbons in the hydrocarbon tail. such bonding reduces the number of hydrogen atoms attached to the carbon skeleton. |  | 24 |
| 11234655323 | fatty acid | a carboxylic acid with a long carbon chain. Vary in length and __________ linked to a glycerol molecule form a fat molecule, also called triglyceride. | 25 | |
| 11234655324 | chitin | a structural polysaccharide, consisting of amino sugar monomers, found in many fungal cell walls and in the exoskeletons of all arthropods. | 26 | |
| 11234655325 | trans fat | an unsaturated fat, formed artificially during hydrogenation of oils, containing one or more trans double bonds. |  | 27 |
| 11234655326 | phospholipid | a lipid made up of glycerol joined to two fatty acids and a phosphate group. The hydrocarbon chains of the fatty acids act as nonpolar hydrophobic tails, while the rest of the molecule acts s a polar, hydrophilic head. They form bilayers that function as biological membrane. |  | 28 |
| 11234655343 | phospholipid bilayer | function as membranes |  | 29 |
| 11234655327 | steroid | a type of lipid characterized by a carbon skeleton consisting of four fused rings with various chemical groups attached. Function as part of membranes or hormones. |  | 30 |
| 11234655328 | catalyst | a chemical agent that selectively increases the rate of a reaction without being consumed by the reaction. | 31 | |
| 11234655329 | hydrophobic | a type of weak chemical interaction caused when molecules that do not mix with water coalesce to exclude water. | 32 | |
| 11234655330 | polypeptide | a polymer of many amino acids linked together by peptide bonds. | 33 | |
| 11234655331 | nucleic acid | a polymer (polynucleotide) consisting of many nucleotide monomers; serves as a blueprint for proteins and, through the actions of proteins, for all cellular activities. the two types are DNA and RNA. | 34 | |
| 11234655344 | functions of nucleic acid | functions as storage, transmission & use of genetic material | 35 | |
| 11234655332 | nucleotide | the building block of a nucleic acid, consisting of a five-carbon sugar covalently bonded to a nitrogenous base and one or more phosphate groups. |  | 36 |
| 11234655333 | polynucleotide | a polymer consisting of many nucleotide monomers in a chain. The nucleotides can be those of DNA or RNA. |  | 37 |
| 11234655345 | RNA | transmission of information, consists of monomers with a ribose sugar and nitrogenous bases cytosine (C), guanine (G), adenine (A) & uracil (U). Single stranded. |  | 38 |
| 11234655334 | DNA | a nucleic acid molecule, usually a double-stranded helix, in which each polynucleotide strand consists of nucleotide monomers with a deoxyribose sugar and the nitrogenous bases adenine (A), cytosine (C), guanine (G), and thymine (T); capable of being replicated and determining the inherited structure of a cell's proteins. |  | 39 |
| 11234655335 | deoxyribose | the sugar component of DNA nucleotides, having one fewer hydroxyl group than ribose, the sugar component of RNA nucleotides. |  | 40 |
| 11234655336 | ribose | the sugar component of RNA nucleotides. |  | 41 |
| 11234655337 | double helix | the form of native DNA, referring to its two adjacent antiparallel polynucleotide strands wound around an imaginary axis into a spiral shape. | 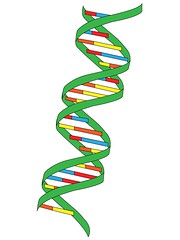 | 42 |
| 11234655346 | amphipathic | Definition. adjective. (1) Pertains to a molecule containing both polar (water-soluble) and nonpolar (not water-soluble) portions in its structure. (2) Of, or relating to, a molecule having hydrophobic and hydrophilic regions | 43 |
Campbell Biology Chapter 5 Flashcards
Primary tabs
Need Help?
We hope your visit has been a productive one. If you're having any problems, or would like to give some feedback, we'd love to hear from you.
For general help, questions, and suggestions, try our dedicated support forums.
If you need to contact the Course-Notes.Org web experience team, please use our contact form.
Need Notes?
While we strive to provide the most comprehensive notes for as many high school textbooks as possible, there are certainly going to be some that we miss. Drop us a note and let us know which textbooks you need. Be sure to include which edition of the textbook you are using! If we see enough demand, we'll do whatever we can to get those notes up on the site for you!

