| 10640532940 | chitin | Polysaccharide (carbohydrate made of multiple sugar molecules) that help strengthen exoskeletons and fungal cell walls |  | 0 |
| 10640532941 | catalyst | substance that speeds up the rate of a chemical reaction | 1 | |
| 10640532942 | double helix | two strands of nucleotides wound about each other; structure of DNA | 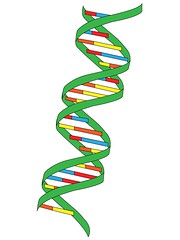 | 2 |
| 10640532943 | enzyme | a type of protein that acts as a catalyst to speed up chemical reactions | 3 | |
| 10640532944 | fat | A lipid composed of glycerol + fatty acids. They store large amounts of energy. | 4 | |
| 10640532945 | fatty acid | lipid that consists of a carboxyl group (OH-C=O) attached to a long carbon skeleton |  | 5 |
| 10640532946 | glycogen | a polysaccharide (carbohydrate with multiple sugars) in animals that stores glucose for energy | 6 | |
| 10640532947 | glycosidic linkage | A covalent bond formed between two monosaccharides (singular sugar molecules) by a dehydration reaction. |  | 7 |
| 10640532948 | hydrolysis | A chemical reaction where the the bonds between two molecules are broken when water is added |  | 8 |
| 10640532949 | lipids | Large biological molecules that do not form polymers and mix poorly with water. Examples includes fats, phospholipids, and steroids. | 9 | |
| 10640532950 | monomer | small chemical unit that makes up a polymer |  | 10 |
| 10640532951 | monosaccharides | A single sugar molecule; the building block of carbohydrates. Their names often end in -ose. Examples include glucose, galactose, and fructose. |  | 11 |
| 10640532952 | nucleic acid | Macromolecules that consist of many linked nucleotides. They store, transmit, and express hereditary information. Examples include DNA and RNA. |  | 12 |
| 10640532953 | nucleotide | Substance that is the building block of nucleic acids (DNA & RNA). Is composed of three parts: a five-carbon sugar (a pentose), a nitrogen-containing (nitrogenous) base, and 1-3 phosphate groups. |  | 13 |
| 10640532954 | peptide bond | The chemical bond formed between two amino acids. Occurs when the carboxyl group (COOH) of one amino acid bonds with the amino group (NH2) of another, forming CONH and releasing water. |  | 14 |
| 10640532955 | polymer | a long molecule consisting of many similar building blocks (monomers) |  | 15 |
| 10640532956 | phospholipid | A lipid made up of glycerol + a phosphate group + two fatty acids. They form bilayers that function as biological membranes. |  | 16 |
| 10640532957 | polysaccharide | Carbohydrates that are made up multiple saccharide (sugar) molecules bonded together. Examples include cellulose & starch (plants), glycogen (animals), chitin (animals and fungi). |  | 17 |
| 10640532958 | protein | Macromolecules comprised of multiple amino acids. | 18 | |
| 10640532959 | purine | Adenine and guanine; nitrogenous bases that have a six-membered ring fused to a five-membered ring |  | 19 |
| 10640532960 | pyrimidine | Cytosine, thymine, and uracil; nitrogenous bases that have a single six-membered ring |  | 20 |
| 10640532961 | ribose | A five-carbon sugar present in RNA |  | 21 |
| 10640532962 | steroids | A type of lipid that contains four fused rings of carbon atoms. Examples include hormones or cholesterol. |  | 22 |
| 10640532963 | trans fat | An unsaturated fat formed artificially during hydrogenation of oils, containing one or more trans double bonds. Can increase the risk of atherosclerosis. |  | 23 |
| 10640532964 | triglyceride | A lipid consisting of glycerol + three fatty acids. They are often found in fats and oils and are an important source of energy. |  | 24 |
| 10640532965 | amino acid | The building blocks of proteins; contains both a carboxyl (COOH) and an amino (NH2) group. |  | 25 |
| 10640532966 | carbohydrates | Group of macromolecules that include sugars and polymers of sugars. They serve as fuel and building material. |  | 26 |
| 10640532967 | cellulose | Polysaccharide (carbohydrate made of multiple sugar molecules) that helps strengthen plant cell walls | 27 | |
| 10640532968 | chaperonin | A protein molecule that assists in the proper folding of other proteins. |  | 28 |
| 10640532969 | dehydration reaction | A chemical reaction in which two molecules become bonded to each other when a water molecule is lost |  | 29 |
| 10640532970 | denaturation | a process where changes in temperature, pH, or exposure to chemicals causes a protein to change shape and become dysfunctional |  | 30 |
| 10640532971 | DNA | - Sugar = deoxyribose - Nitrogenous bases = C, G, A, T - Usually double-stranded - Stores hereditary information |  | 31 |
| 10640532972 | disaccharides | Double sugars; carbohydrates made of two monosaccharides (sugar molecules) joined by a covalent bond. Examples include sucrose, maltose, and lactose. |  | 32 |
| 10640532973 | macromolecules | A giant molecule formed by the joining of smaller molecules. Carbohydrates, proteins, and nucleic acids are examples. | 33 | |
| 10640532974 | RNA | - Sugar = ribose - Nitrogenous bases = C, G, A, U - Usually single-stranded - Various functions in gene expression, including carrying instructions from DNA to ribosomes |  | 34 |
| 10640532975 | starch | a polysaccharide (carbohydrate with multiple sugars) in plants that stores glucose for energy | 35 | |
| 10640532976 | saturated fatty acid | A fatty acid in which the fatty acid chains have mostly single bonds. They are solid at room temperature |  | 36 |
| 10640532977 | unsaturated fatty acid | A fatty acid with at least one double bond. They are liquid at room temperature. |  | 37 |
| 10640532978 | primary structure | sequence of amino acids, forming a protein chain |  | 38 |
| 10640532979 | secondary structure | 39 | ||
| 10640532980 | alpha helix | 40 | ||
| 10640532981 | beta pleated sheet | 41 | ||
| 10640532982 | tertiary structure | 42 |
AP Biology: Chapter 5 Flashcards
Primary tabs
Need Help?
We hope your visit has been a productive one. If you're having any problems, or would like to give some feedback, we'd love to hear from you.
For general help, questions, and suggestions, try our dedicated support forums.
If you need to contact the Course-Notes.Org web experience team, please use our contact form.
Need Notes?
While we strive to provide the most comprehensive notes for as many high school textbooks as possible, there are certainly going to be some that we miss. Drop us a note and let us know which textbooks you need. Be sure to include which edition of the textbook you are using! If we see enough demand, we'll do whatever we can to get those notes up on the site for you!

