| 12596412180 | anabolic | synthesis-build up reactions- | 0 | |
| 12596412181 | catalysis | the acceleration of a chemical reaction by a catalyst. | 1 | |
| 12596412182 | chemical bonds | Represent stored energy |  | 2 |
| 12596412183 | Reduction reaction | In chemistry- The gain of electrons |  | 3 |
| 12596412184 | Oxidation reactions | The loss of electrons | 4 | |
| 12596412185 | (CH4 or CO2) which one is more highly reduced and which is more highly oxidized | -CH4 is more highly reduced- has a lot of reducing power -CO2 Is more highly oxidized- has little reducing power?????????????? | 5 | |
| 12596412186 | Electrochemical gradients | gradient is the difference in concentration - Difference of electron concentration in compared to one place to another - Always flow from high to low energy (*think of battery) | 6 | |
| 12596412187 | ATP (Adenosine Triphosphate) | -when cellular work needs to be done the cell uses ATP - High amounts of energy are stored in The extremely rich peptide bonds |  | 7 |
| 12596412188 | Are enzymes written into chemical equations? If so where... | they are but written on the arrow separating the equations | 8 | |
| 12596412189 | Active sites | The part of the enzyme that actually does the job |  | 9 |
| 12596412190 | What is the chemistry term for enzyme? | catilist | 10 | |
| 12596412191 | Are enzymes consumed during the reaction | No they act as a "middle man" Reactants get attached, a product is formed , the reactants the detach | 11 | |
| 12596412192 | what does an enzymes relies on to do its job | Macanics and probability - Enzymes require the components to be in the correct orientation to create a product | 12 | |
| 12596412193 | How can an enzyme function be controlled | use an inhibitor | 13 | |
| 12596412194 | (Ea) | Activation energy- The energy required to start a reaction - The energy required to contort the reactant molecules so the bonds can break - The energy required to push the reactants to the top of an energy barrier so the downhill part of the reaction can began - activation energy is often supplied by heat in the form of thermal energy that the reactant molecules absorb from there surroundings - The absorbtion of thermal energy accelerates accelerates the reactant molecules so they colide more and more forcefully |  | 14 |
| 12596412195 | enzymes do what to the Ea of a reaction | They decrease the (Ea) barrier by hundreds of thousands of times. allowing the molecules to absorb enough energy to reach the transition state even at moderate temeratures | 15 | |
| 12596412196 | After en enzyme is used once what happens to it | The enzyme stays intact and on goes the reactios | 16 | |
| 12596412197 | phosphate group | On the end of ATP and ADP - Releces a high amount of energy when broken away from the ATP because of H2O. - extremely elextronegative -phosphate bonds are referred to as high energy phosphate bonds |  | 17 |
| 12596412198 | The cells proteans harnis the energy releced by atp in several ways | ATP is used to drive chemical reactions - transpertation and michanical work are almost nearly powered by atp | 18 | |
| 12596412199 | what drives most celular work | Chemical potential energy stored in ATP drives most cellular work | 19 | |
| 12596412200 | Where does the free energy required to Phosphorylate ADp come from | It comes from the exergonic breakedown reactions called Catabolism in the cell (such as cellular respiration) - The atp cycle accures at an astonishing rate, muschle group can recycle all its atp in less then a minute. | 20 | |
| 12596412201 | Active Site | The part of the enzime that does the job of arranging the substrate |  | 21 |
| 12596412202 | the substrate | the substrate is the molecule that fits into the enzyme | 22 | |
| 12596412203 | induced fit | the enzime cups the substrate when its inside |  | 23 |
| 12596412204 | cofactor or coenzyme | -helps enzimes -non-protean helpers that meny enzymes require, sometimes copper, zinc or iron | 24 | |
| 12596412205 | inhibitors | Inhibit with the actions of a specific enzyme | 25 | |
| 12596412206 | competitive inhibitors | reduce the productivity of enzymes by blocking substrates from entering the active sites so they become less available |  | 26 |
| 12596412207 | noncompetitive inhibitor | do not directly compete with the substrates to bind to the enzyme at the active sites but incited bind to the enzyme in other places causing it to change its shape becoming less effective at catolosis |  | 27 |
| 12596412208 | whats a waste product of respiration | carbon Dioxide | 28 | |
| 12596412209 | energy flows in as sunlight and leaves as >>> | heat!!!! | 29 | |
| 12596412210 | what are catabolic pathways | Catabolic pathways are medibolic pathways that releace storred energy by breaking down complex molecules | 30 | |
| 12596412211 | fermentation | a catabolic process that accrues without oxygen - not the most efficient |  | 31 |
| 12596412212 | Aerobic Resperation | The most effective catibolic pathway - oxygen is consumed as a reactant along with organic fuel | 32 | |
| 12596412213 | What is the point of glycolosis | It is to produce more ATP and NADH | 33 | |
| 12596412214 | Glycolysis | -Glycolysis in which the carbohydrate Glucose is oxidized - ATP is produced & NAD is reduced to NADH | 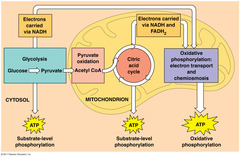 | 34 |
| 12596412215 | Where does Glycolysis occure | Glycolysis Occurs in the cytosol | 35 | |
| 12596412216 | Glucose | A highly reduced molecule -RICH in potential energy |  | 36 |
| 12596412217 | NAD^+ | A Coenzyme, (electron carrier ) -NAD^+ goes to NADH |  | 37 |
| 12596412218 | Glycolysis Consumes ATP to produce ATP???? WHY | For every molecule of glucose metabolized via Glycolysis -2 ATP Molecules are produced -2 NAD Molecules are reduced (2NAD->2NADH) -2 Pyruvate molecules remain | 38 | |
| 12596412219 | metabolism | the sum product of the anabolic and catabolic processes in a living system | 39 | |
| 12596412220 | anabolic | building up | 40 | |
| 12596412221 | catabolic | braking down |  | 41 |
| 12596412222 | how can energy be stored in a biological system | - chemical bonds -reducing power -electrochemical gradiants | 42 | |
| 12596412223 | Krebs Cycle | - occures in matrex of mitocondra - produces lots of ATP |  | 43 |
| 12596412224 | in anerobic cells the electrons alternately get accepted by | O2 | 44 | |
| 12596412225 | ATP Sinthase | Carries the reaction in the mitocondra which converts ADP to ATP | 45 |
Champbell's Bio Tenth ed. chapters 8-9 Flashcards
Primary tabs
Need Help?
We hope your visit has been a productive one. If you're having any problems, or would like to give some feedback, we'd love to hear from you.
For general help, questions, and suggestions, try our dedicated support forums.
If you need to contact the Course-Notes.Org web experience team, please use our contact form.
Need Notes?
While we strive to provide the most comprehensive notes for as many high school textbooks as possible, there are certainly going to be some that we miss. Drop us a note and let us know which textbooks you need. Be sure to include which edition of the textbook you are using! If we see enough demand, we'll do whatever we can to get those notes up on the site for you!

