AP Bio Ch. 3, 4, 5, & 6 Notes/Practice Tes/Quiz Bowl Review Flashcards
Notes compressed, then put into a quizlet review. Everything in here is from AP Bio book. Also Practice Test and Quiz Bowl.
| 943912526 | Molecules of life containing the element carbon and at least one hydrogen atom. | Organic Compound | 1 | |
| 943912527 | Consist only of hydrogen atoms covalently bonded to carbon. | Hydrocarbon | 2 | |
| 943912528 | Particular atoms or clusters of atoms covalently bonded to carbon. | Functional Group | 3 | |
| 943912529 | Cells use individual subunits. | Monomer | 4 | |
| 943912530 | Consist of three to millions of subunits that may or may not be identical. | Polymer | 5 | |
| 943912531 | Belong to a class of organic compounds, the alcohols. | Sugar | 6 | |
| 943912532 | Can split molecules or join them. | Enzyme | 7 | |
| 943912533 | Enzymes split an -OH group from one molecule and an H atom from another. The discarded atoms often form water. | Condensation Reaction | 8 | |
| 943912534 | Like condensation in reverse. | Hydrolysis | 9 | |
| 943912535 | Are the simplest carbohydrates. | Monosaccharide | 10 | |
| 943912536 | Are the sugar monomers of RNA and DNA. | Ribose and Deoxyribose | 11 | |
| 943912537 | Cells use it as an instant energy source. | Glucose | 12 | |
| 943912538 | A short chain of covalently bonded sugar monomers. | Oligosaccharide | 13 | |
| 943912539 | Consist of two sugar monomers. | Disaccharide | 14 | |
| 943912540 | The most plentiful sugar in nature, has a glucose and a fructose unit. | Sucrose | 15 | |
| 943912541 | Are straight or branched chains of many sugar monomers. | Polysaccharide | 16 | |
| 943912542 | The sugar-storage equivalent of starch in plants. | Glycogen | 17 | |
| 943912543 | A modified polysaccharide. Strengthens external skeleton. | Chitin | 18 | |
| 943912544 | Used as structural materials, others as packets of instant energy, and others as transportable or storable forms of energy. | Carbohydrate | 19 | |
| 943912545 | Being non-polar hydrocarbons, they do not dissolve in water. | Lipid | 20 | |
| 943912546 | Lipids with one, two, or three fatty acids dangling like tails. | Fats | 21 | |
| 943912547 | Starts as a carboxyl group attached to as many as thirty-six carbon atoms. | Fatty Acid | 22 | |
| 943912548 | Contain one or more double covalent bonds. | Unsaturated Fatty Acid | 23 | |
| 943912549 | Have single covalent bonds only. | Unsaturated Fatty Acid | 24 | |
| 943912550 | three fatty acid tails linked to one glycerol. Most abundant lipid in your body, richest reservoir of energy. (Neutral) | Triglyceride | 25 | |
| 943912551 | Have a glycerol backbone, two non-polar fatty acid tails, and a polar head. Main components of cell membranes. | Phospholipid | 26 | |
| 943912552 | Long chain fatty acids tightly packed and bonded to long-chain alcohols or carbon rings. | Wax | 27 | |
| 943912553 | Lipids with no fatty acids. Differ in the number, position, and type of their functional groups. All have a rigid backbone of four fused-together carbon rings. | Sterol | 28 | |
| 943912554 | A small organic compound with an amino group, a carboxyl group, a hydrogen atom, and one or more atoms called its R group. | Amino Acid | 29 | |
| 943912555 | Forms as a condensation reaction joins the amino group of one amino acid and the carboxyl group of the next in line. | Peptide Bond | 30 | |
| 943912556 | Consists of three or more amino acids. | Polypeptide Chain | 31 | |
| 943912557 | They consist of two or more polypeptide chains. | Quaternary Structure | 32 | |
| 943912558 | A polypeptide chain or parts of it become organized as structurally stable, compact, functional domains. | Tertiary Structure | 33 | |
| 943912559 | A protein's shape and charge distribution around that shape dictate _____. | Protein Function | 34 | |
| 943912560 | The cholesterol, triglycerides, and phospholipids that your body absorbs after a meal are transported about as components. | Lipoprotein | 35 | |
| 943912561 | Often attach short, linear, or branched oligosaccharides to a new polypeptide chain, making a glycoprotein. | Enzyme | 36 | |
| 943912562 | Have one sugar, at least one phosphate group, and one nitrogen-containing base. Deoxyribose or ribose is the sugar. | Nucelotide | 37 | |
| 943912563 | Has a row of three phosphate groups attached to its sugar. | ATP | 38 | |
| 943912564 | Necessary for enzyme function. | Coenzyme | 39 | |
| 943912565 | Contains a large vacuole that reduces the volume of the cytoplasm. | Plant Cell | 40 | |
| 943912566 | A lipid is ___________ in water, an important constituent in cell membranes, and contain twice as much energy as an equivalent weight of polysaccharide. | Insoluble | 41 | |
| 943912567 | ___________ or ___________ cells have enzymes, DNA, ribosome's, plasma membrane, and mitochondria. | Plant Animal | 42 | |
| 943912568 | An organism with a cell call would have the most difficulty doing which process? | Phagocytosis | 43 | |
| 943912569 | When a molecule has double bonds between Carbon atoms it is a ___________ fatty acid. | Unsaturated | 44 | |
| 943912570 | The A helix and the B pleated sheet are both common polypeptide forms found in which level of protein structure? | Secondary | 45 | |
| 943912571 | The 20 different amino acids found in polypeptides exhibit different chemical and physical properties because different ___________ chains and R groups. | Side | 46 | |
| 943912572 | Flow of Information in eukaryotic ___________ to ___________ to Proteins. (2) | DNA RNA | 47 | |
| 943912573 | Produces and modifies polysaccharides that will be secreted. | Golgi Apparatus | 48 | |
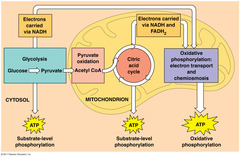
| 943912574 | Contains its own DNA and Ribosomes. | Mitochondria | 49 | |
| 943912575 | Contains hydrolytic enzymes. | Lysosome | 50 | |
| 943912576 | One of the main energy transformers of cells. | Mitochondria | 51 | |
| 943912577 | Sugar in DNA vs Sugar in RNA is different because Sugar in DNA contains one less ___________ atom. | Oxygen | 52 | |
| 943912578 | Which is not found in DNA? Thymine, Adenine, Uracil, Guanine, and Cytosine. | Uracil | 53 | |
| 943912579 | Grana, Thylakoids, and Stroma are found in the ___________. | Chloroplast | 54 | |
| 943912580 | The movement of a substance across a biological membrane against it's concentration gradient with the help of an energy input is ___________. | Active Transport | 55 | |
| 943912581 | Plasma Membrane is found in ___________ cells. | All | 56 | |
| 943912582 | ___________ Bonds are created during the formation of the primary structure. | Peptide | 57 | |
| 943912583 | Motor proteins are associated with ___________ when providing molecular motion. | Cytoskeleton | 58 | |
| 943912584 | A polypeptide is a ___________ containing a total of 20 peptide bonds. | Polymer | 59 | |
| 943912585 | Polysaccharides contain ___________, starch, and chitin. | Glycogen | 60 | |
| 943912586 | When something becomes limp and soft it is ___________. | Hypotonic | 61 | |
| 943912587 | When something remains hard and stiff it is ___________. | Hypertonic | 62 | |
| 943912588 | When the solution is equal to each side, the tonicity is ___________. | Isotonic | 63 | |
| 943912589 | Enzyme ___________ is dependent on the pH and temperature of the reaction environment. | Catalysis | 64 | |
| 943912590 | Enzymes catalysis is dependent of the ___________ structure or conformation of the enzyme. | Three-Dimensional | 65 | |
| 943912591 | Enzymes (do/don't) provide activation energy for the reaction they catalyze. | don't | 66 | |
| 943912592 | A ___________ ___________ must be removed so two amino acids can bond to form a larger molecule. | Water Molecule | 67 | |
| 943912593 | Cell Wall provides support and protection. (T/F) | T | 68 | |
| 943912594 | Chloroplasts are sites of cellular respiration. (T/F) | F | 69 | |
| 943912595 | Chromosomes provide genetic control information. (T/F) | T | 70 | |
| 943912596 | Ribosomes are the site of protein synthesis. (T/F) | T | 71 | |
| 943912597 | Mitochondria function as the formation of ATP. (T/F) | T | 72 | |
| 943912598 | What are the types of molecules that are the major structural components of the cell membrane? Phospholipids and ___________. | Protein | 73 | |
| 943912599 | What kinds of molecules pass through a cell membrane most easily? Small and ___________. | Hydrophobic | 74 | |
| 943912600 | Breaking down large molecules into smaller ones is called ___________. | Catabolism | 75 | |
| 943912601 | Fungi is a prokaryotic cell. (T/F) | F | 76 | |
| 943912602 | When the end product of a metabolic pathway inhibits an earlier step in the pathway is ___________ ___________. | Feedback Inhibition | 77 | |
| 943912603 | Enzymes may require a nonprotein cofactor or ion for catalysis to take place. (T/F) | T | 78 | |
| 943912604 | Enzyme function is reduced if the three-dimensional structure or conformation of an enzyme is altered. (T/F) | T | 79 | |
| 943912605 | Enzyme function is influenced by physical and chemical environmental factors such as pH and temperature. (T/F) | T | 80 | |
| 943912606 | An animal cell (without oligosaccharides) can not ___________-___________ ___________. | Cell Cell Recognition | 81 | |
| 943912607 | When a protein's three dimensional shape or conformation changes due to disruption in hydrogen bonds, disulfide bridges, or ionic bonds, it is called ___________. | Denaturation | 82 | |
| 943912608 | Both starch and ___________ are polymers of glucose. | Cellulose | 83 | |
| 943912609 | Prokaryotic cells have DNA, a cell wall, a plasma membrane, and an ER. (T/F) | F | 84 | |
| 943912610 | Changing any level of structural organization can change the function of a protein. (T/F) | T | 85 | |
| 943912611 | A nonprotein that is a helper of an enzyme molecule is called ___________. | Coenzyme | 86 | |
| 943912612 | ___________ Pathways consume energy to build up polymers from monomers. | Anabolic | 87 | |
| 943912613 | Anabolic pathways are ___________ regulated sequences of chemical reactions. | Highly | 88 | |
| 943912614 | Membrane phospholipids are able to ___________ laterally along the plane of the plasma ___________. | Move Membrane | 89 | |
| 943912615 | When it exhibits a specificity for a particular type of molecule it is a ___________ protein. | Carrier | 90 | |
| 943912616 | ___________ are able to convert light energy to chemical energy. | Chloroplasts | 91 | |
| 943912617 | Typical animals cells are a ___________ solution. | Isotonic | 92 | |
| 943912618 | Plants cells are typically a ___________ solution. | Hypotonic | 93 | |
| 943912619 | A passive process in which molecules move from a region of higher concentration to a region of lower concentration is called ___________. | Diffusion | 94 | |
| 943912620 | ___________ membrane proteins can perform active transport, hormone reception, cell adhesion, and ___________ attachment. | Integral Cytoskeleton | 95 | |
| 943912621 | 1:2:1 ratio of Carbon, Hydrogen, and Oxygen is a ___________. | Carbohydrate | 96 | |
| 943912622 | Nucleotides have a ___________ base, and ___________ group, and a pentose sugar. | Nitrogenous Phosphate | 97 | |
| 943912623 | ___________ does not take material into cells. | Exocytosis | 98 | |
| 943912624 | Pinocytosis, endocytosis, active transport, and carrier facilitated diffusion all take materials into cells. (T/F) | T | 99 | |
| 943912625 | ___________ Bonds maintain the secondary structure of a protein. | Hydrogen | 100 | |
| 943912626 | Saturated fatty acids have a ___________ ratio of hydrogen to carbon than do unsaturated fatty acids. | Higher | 101 | |
| 943912627 | In the double helix structure of nucleic acids, cytosine hydrogen bonds to ___________. | Guanine | 102 | |
| 943912628 | In the double helix structure of nucleic acids, ___________ hydrogen bonds to guanine. | Cytosine | 103 | |
| 943912629 | Metabolism is an emergent property of life at the level of organisms. (T/F) | F | 104 | |
| 943912630 | Metabolism depends on an infrequent supply of energy. (T/F) | F | 105 | |
| 943912631 | Thermodynamic barrier must be overcome to form products, this is the ___________ energy. | Activation | 106 | |
| 943912632 | Enzyme reactions are faster than the same reaction in the absence of the enzyme. (T/F) | T | 107 | |
| 943912633 | Enzymes ___________ the rate of a reaction. | Increase | 108 | |
| 943912634 | Enzymes are permanently altered by the reactions they catalyze. (T/F) | F | 109 | |
| 943912635 | 9+2 arrangement of microtubules is in cilia, ___________, and flagella. | Centrioles | 110 | |
| 943912636 | ___________ ___________ are not part of the cell membrane. | Nucleic Acids | 111 | |
| 943912637 | Steroids are not part of the cellular membrane. (T/F) | F | 112 | |
| 943912638 | The two strands making up the DNA double helix molecule are held together by ___________ bonds. | Hydrogen | 113 | |
| 943912639 | Plasmodesmata in plant cells are most similar in function to ___________ junctions. | Gap | 114 | |
| 943912640 | Carbonyl groups are ___________ atoms joined to an oxygen by a ___________ covalent bond. | Carbon Double | 115 | |
| 943912641 | Endergonic reactions require a net ___________ of energy. | Input | 116 | |
| 943912642 | Do cytoskeletons maintain a critical limit of cell size? (Yes/No) | No | 117 | |
| 943912643 | ___________ is a major structural component of ___________ cells. | Cellulose Plant | 118 | |
| 943912644 | Integral membrane proteins can perform protein synthesis. (T/F) | F | 119 | |
| 943912645 | Processes take material into cells except for ___________. | Exocytosis | 120 | |
| 943912646 | Where in cells are proteins manufactured? ___________ ___________ ___________. | Rough Endoplasmic Reticulum | 121 | |
| 943912647 | Tertiary structure of a protein is the unique 3D shape of the fully ___________ ___________. | Folded Polypeptide | 122 | |
| 943912648 | Functional Groups always found in amino acids? ___________ and ___________. | Carboxyl Amino | 123 | |
| 943912649 | In enzyme catalysis, the ___________ of the ___________ occurs. | Binding Substrate | 124 | |
| 943912650 | Redox reactions often ___________ H+. | Releases | 125 | |
| 943912651 | In electron transfer chains, one molecule is ___________, the next is reduced. | Oxidized Reduced | 126 | |
| 943912652 | Temperature, pH, Salt Concentration, Allosteric regulators, and cofactors are all factors influencing enzyme activity. (T/F) | T | 127 | |
| 943912653 | Small increase in temperature increases reaction rates. (T/F) | T | 128 | |
| 943912654 | Low temperatures disrupt bonds and destroy the shape of the active site. (T/F) | F | 129 | |
| 943912655 | At equilibrium, amount of reactant seldom equals amount of product. (T/F) | T | 130 | |
| 943912656 | Metabolic pathways are ___________ (anabolic) and ___________ (catabolic). | Biosynthetic Degradative | 131 | |
| 943912657 | Enzymes inducing fit between enzyme and ___________. | Substrate | 132 | |
| 943912658 | Allosteric Activation/___________ is when it may open active site or close it. | Inhibition | 133 | |
| 943912659 | ___________ is the measure of degree of disorder in a system. | Entropy | 134 | |
| 943912660 | Intermediates are participants in metabolic reactions. So are cofactors and transport proteins. (T/F) | T | 135 | |
| 943912661 | RNA has ___________ in place of thymine. | Uracil | 136 | |
| 943912662 | Glycoproteins are proteins combined with ___________. | Oligosaccharides | 137 | |
| 943912663 | Denaturation is the breakage of ___________ bonds. | Weak | 138 | |
| 943912664 | ___________s are energy carriers, coenzymes, chemical messengers, and building blocks for nucleic acids. | Nucleotide | 139 | |
| 943912665 | ___________ Structure is multiple polypeptide chains. | Quaternary | 140 | |
| 943912666 | ___________ Structure is when H+ bonds often created pleated pattern. | Secondary | 141 | |
| 943912667 | Peptide bonds are when amino groups of one amino acids links with ___________ group of next | Carboxyl | 142 | |
| 943912668 | Waxes are ___________ chain fatty acids linked to long chain alcohols. | Long | 143 | |
| 943912669 | Sterols and Derivatives (do/don't) have fatty acids. They also (do/don't) have a rigid backbone of four fused carbon rings. | Don't Do | 144 | |
| 943912670 | Amino acids maybe polar or non-polar. They can only be uncharged. (T/F) | F | 145 |