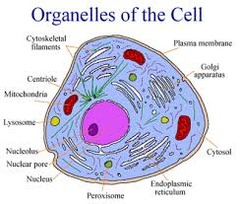
| 8940787204 | Acid | compound that donates a proton (H+) when dissolved in a solution. | | 0 |
| 8940787205 | Adhesion | force of attraction between different kinds of molecules |  | 1 |
| 8940787206 | Antiparallel | The opposite arrangement of the sugar-phosphate backbones in a DNA double helix.
The opposite arrangement of the sugar-phosphate backbones in a DNA double helix. |  | 2 |
| 8940787207 | Archaea | Microorganisms that live in extreme environments. (resemble bacteria but are not bacteria) |  | 3 |
| 8940787208 | Atom | The smallest particle of an element |  | 4 |
| 8940787209 | Atomic Mass | Number of protons and neutrons |  | 5 |
| 8940787210 | Atomic Nucleus | An atom's dense central core, containing protons and neutrons.
An atom's dense central core, containing protons and neutrons. |  | 6 |
| 8940787211 | Atomic Number | |  | 7 |
| 8940787212 | ATP (Adenosine Triphosphate) | main energy source that cells use for most of their work, energy currency for life |  | 8 |
| 8940787213 | Base | A substance that decreases the hydrogen ion concentration in a solution. | | 9 |
| 8940787214 | Buffer | mixtures that can react with acids or bases to keep the pH within a particular range |  | 10 |
| 8940787215 | Calorie | Amount of energy needed to raise temperature 1 gram of water 1 degree C |  | 11 |
| 8940787216 | Catalyst | substance that speeds up the rate of a chemical reaction |  | 12 |
| 8940787217 | Celsius | the metric temperature scale in which water freezes at 0 degrees and boils at 100 degrees. |  | 13 |
| 8940787218 | Chemical Bond | the attractive force that holds atoms or ions together |  | 14 |
| 8940787219 | Chemical Equilibrium | the rate of formation of products equals the rate of formation of reactants |  | 15 |
| 8940787220 | Chemical Reaction | A change in which one or more substances are converted into new substances. |  | 16 |
| 8940787221 | Chitin | Polysaccharide found in arthropod exoskeletons and fungal cell walls. |  | 17 |
| 8940787222 | Cis/Trans Isomer | Cis-trans isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are rotated into a different orientation in three-dimensional space. |  | 18 |
| 8940787223 | Cohesion | Attraction between molecules of the same substance |  | 19 |
| 8940787224 | Compound | A substance made up of atoms of two or more different elements joined by chemical bonds |  | 20 |
| 8940787225 | Controlled Experiment | An experiment in which only one variable is manipulated at a time. |  | 21 |
| 8940787226 | Covalent Bond | |  | 22 |
| 8940787227 | Deoxyribonucleic Acid | (DNA) nucleic acid that contains the sugar deoxyribose |  | 23 |
| 8940787228 | Double Bond | a covalent bond produced by the sharing of two pairs of electrons between two atoms |  | 24 |
| 8940787229 | Double Helix | The shape of a DNA molecule |  | 25 |
| 8940787230 | Energy | the ability to do work
the capacity to cause change |  | 26 |
| 8940787231 | Enzyme | protein catalyst that speeds up the rate of specific biological reactions |  | 27 |
| 8940787232 | Essential Element | A chemical element required for an organism to survive, grow, and reproduce |  | 28 |
| 8940787233 | Eukaryotic Cell | cell that has a nucleus and other membrane-bound organelles.
cell that has a nucleus and other membrane-bound organelles. |  | 29 |
| 8940787234 | Evaporative Cooling | A property of water whereby a body becomes cooler as water evaporates from it. |  | 30 |
| 8940787235 | Evolution | The process through which species change over time |  | 31 |
| 8940787236 | Fat | A class of energy-giving nutrients; also the main form of energy storage in the body |  | 32 |
| 8940787237 | Fatty Acid | hydrocarbon chain often bonded to glycerol in a lipid |  | 33 |
| 8940787238 | Functional Group | An atom or group of atoms that determine the functions or properties of the compound |  | 34 |
| 8940787239 | Genomics | study of whole genomes, including genes and their functions |  | 35 |
| 8940787240 | Geometric Isomer | isomer that differs in the placement of groups around a double bond; cis/trans isomerism |  | 36 |
| 8940787241 | Glycogen | animal starch
Extremely branched polymer of glucose. |  | 37 |
| 8940787242 | Glycosidic Linkage | A covalent bond formed between two monosaccharides by a dehydration reaction. |  | 38 |
| 8940787243 | Heat | the transfer of thermal energy |  | 39 |
| 8940787244 | Homeostasis | A tendency to maintain a balanced or constant internal state
A tendency to maintain a balanced or constant internal state |  | 40 |
| 8940787245 | Hydrocarbon | Compounds composed of only carbon and hydrogen |  | 41 |
| 8940787246 | Hydrogen Bond | a weak interaction involving a hydrogen atom and a fluorine, oxygen, or nitrogen atom |  | 42 |
| 8940787247 | Hydrolysis | the chemical breakdown of a compound due to reaction with water. |  | 43 |
| 8940787248 | Hypothesis | A testable prediction, often implied by a theory |  | 44 |
| 8940787249 | Inquiry | an official investigation or formal series of questions |  | 45 |
| 8940787250 | Ion | An atom or group of atoms that has a positive or negative charge. |  | 46 |
| 8940787251 | Ionic Bond | A chemical bond resulting from the attraction between oppositely charged ions. |  | 47 |
| 8940787252 | Isomer | Compounds that have the same chemical formula but different three-dimensional structures |  | 48 |
| 8940787253 | Isotope | Atoms of the same element that have different numbers of neutrons |  | 49 |
| 8940787254 | Joule | SI unit of energy |  | 50 |
| 8940787255 | Kinetic Energy | the energy an object has due to its motion |  | 51 |
| 8940787256 | Law | a summary of many experimental results and observations; a law tells how things work |  | 52 |
| 8940787257 | Lipid | macromolecule made mostly from carbon and hydrogen atoms; includes fats, oils, and waxes |  | 53 |
| 8940787258 | Matter | Anything that has mass and takes up space |  | 54 |
| 8940787259 | Model Organism | Mice, fruit flies, worms - organisms we use to understand biology because they are easy to maintain and have short generation times |  | 55 |
| 8940787260 | Molarity | the number of moles of solute in one liter of solution |  | 56 |
| 8940787261 | Mole | 6.02x10^23
The SI unit for measuring the amount of a substance |  | 57 |
| 8940787262 | Molecular Mass | The sum of the masses of all the atoms in a molecule; sometimes called molecular weight. |  | 58 |
| 8940787263 | Monomer | a molecule that can be bonded to other identical molecules to form a polymer. |  | 59 |
| 8940787264 | Monosaccharide | A single sugar molecule such as glucose or fructose, the simplest type of sugar. |  | 60 |
| 8940787265 | Natural Selection | A natural process resulting in the evolution of organisms best adapted to the environment. |  | 61 |
| 8940787266 | Negative Feedback | A response to changing conditions that acts to dampen or stop a system's reaction. |  | 62 |
| 8940787267 | Neutron | A subatomic particle that has no charge and that is found in the nucleus of an atom |  | 63 |
| 8940787268 | Nonpolar Covalent Bond | a covalent bond in which the electrons are shared equally by the two atoms |  | 64 |
| 8940787269 | Nucleic Acid | polymer of nucleotides; the genetic material of organisms. |  | 65 |
| 8940787270 | Nucleotide | monomer of nucleic acids (DNA and RNA) |  | 66 |
| 8940787271 | Orbital | A region of space around the nucleus where an electron is likely to be found. |  | 67 |
| 8940787272 | Organic Chemistry | the study of carbon compounds |  | 68 |
| 8940787273 | Peptide Bond | The covalent bond between two amino acid units, formed by a dehydration reaction. |  | 69 |
| 8940787274 | Phospholipid | a lipid that contains phosphorus and that is a structural component in cell membranes |  | 70 |
| 8940787275 | Polar Covalent Bond | A covalent bond in which electrons are not shared equally |  | 71 |
| 8940787276 | Polymer | A covalent compound made up of many small, repeating units linked together in a chain. |  | 72 |
| 8940787277 | Polypeptide | A polymer (chain) of many amino acids linked together by peptide bonds. |  | 73 |
| 8940787278 | Polysaccharide | A carbohydrate polymer of many monosaccharides linked by dehydration reactions |  | 74 |
| 8940787279 | Positive Feedback | A control mechanism that amplifies rather than reduces the stimulus |  | 75 |
| 8940787280 | Potential Energy | energy that results from the position or shape of an object |  | 76 |
| 8940787281 | Prokaryotic Cell | cell lacking a nucleus and most other organelles (bacteria and archeae) |  | 77 |
| 8940787282 | Protein | A three dimensional polymer made of monomers of amino acids. |  | 78 |
| 8940787283 | Purine | double-ring nitrogenous base
Adenine and Guanine |  | 79 |
| 8940787284 | Pyrimidine | single-ring nitrogenous base
Cytosine and Thymine and Uracil |  | 80 |
| 8940787285 | Reactant | a substance that takes part in and undergoes change during a reaction. |  | 81 |
| 8940787286 | Ribonucleic Acid | RNA
Nucleotide monomers that contain the sugar ribose |  | 82 |
| 8940787287 | Ribose | sugar in RNA |  | 83 |
| 8940787288 | Saturated Fatty Acid | Those where all carbons are filled with maximum number of hydrogen atoms |  | 84 |
| 8940787289 | Science | An organized way of gathering and analyzing evidence about the natural world. |  | 85 |
| 8940787290 | Sickle-Cell Anemia | Condition where red blood cells assume an abnormal sickle shape |  | 86 |
| 8940787291 | Single Bond | a covalent bond in which two atoms share one pair of electrons |  | 87 |
| 8940787292 | Solute | the substance that is dissolved |  | 88 |
| 8940787293 | Solution | A homogeneous mixture of two or more substances |  | 89 |
| 8940787294 | Solvent | In a solution, the substance in which the solute dissolves. |  | 90 |
| 8940787295 | Specific Heat | the amount of heat necessary to increase the temperature of a substance |  | 91 |
| 8940787296 | Steroid | large family of chemical substances found in many drugs, hormones, and body components |  | 92 |
| 8940787297 | Structural Isomers | differ in the covalent arrangements of their atoms |  | 93 |
| 8940787298 | Surface Tension | A measure of how difficult it is to stretch or break the surface of a liquid that results from the cohesion of water molecules at the surface of a container of water |  | 94 |
| 8940787299 | Temperature | A measure of the average energy of motion of the particles of a substance. |  | 95 |
| 8940787300 | Theory | a well-tested explanation for a broad set of observations |  | 96 |
| 8940787301 | Trace Element | found in very small amounts in the body
ex- calcium, iron, iodine |  | 97 |
| 8940787302 | Triacylglycerol | three fatty acids linked to one glycerol molecule. |  | 98 |
| 8940787303 | Triple Bond | A chemical bond formed when atoms share three pairs of electrons |  | 99 |
| 8940787304 | Unsaturated Fatty Acid | a fatty acid that lacks some hydrogen atoms and has one or more points of unsaturation |  | 100 |
| 8940787305 | Valence Electron | Electrons in the outermost energy shell of an atom |  | 101 |