AP Biology - Cells Flashcards
Campbell's Ch 6, 7: structure and function, prokaryotes v eukaryotes, mvmnt of substances, protein function. Also Cliff's AP Bio 4th ed., Ch 3: Cells.
| 4975729553 | plasma membrane | separates internal metabolic event; controls mvmnt of materials |  | 0 |
| 4975729554 | saturated fatty acid membrane | packed tight; rigid membrane |  | 1 |
| 4975729555 | unsaturated fatty acid membrane | not packed tight; flexible membrane |  | 2 |
| 4975729556 | selectively permeable | allows: small, unchanged, polar molecules; hydrophobic molecules blocks: large polar molecules; ions |  | 3 |
| 4975729557 | integral proteins | imbedded in bilipid layer |  | 4 |
| 4975729558 | peripheral proteins | attached to membrane surface |  | 5 |
| 4975729559 | channel proteins | passage for hydrophillic substances |  | 6 |
| 4975729560 | aquaporins | channel proteins; increase rate of H20 passage |  | 7 |
| 4975729561 | ion channels | ions; gated channels |  | 8 |
| 4975729562 | gated channels | open and close in response to stimuli EX: nerve and muscle cells |  | 9 |
| 4975729563 | carrier proteins | specific molecules bind, changing protein shape |  | 10 |
| 4975729564 | transport proteins | use ATP (active transport) EX: sodium-potassium pump |  | 11 |
| 4975729565 | recognition proteins | unique identification; glycoproteins EX: blood types |  | 12 |
| 4975729566 | receptor proteins | provide binding sites; activates specific cell response |  | 13 |
| 4975729568 | cholesterol | stability to animal cells; @ high T=maintain firmness, @ low T=allows flexibility |  | 14 |
| 4975729569 | organelles | bodies within cytoplasm; chemical rxns isolated, able to take place w/o interference; large surface areas to max. space for rxns | 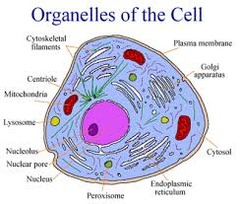 | 15 |
| 4975729570 | nucleus | contains DNA and nucleoli; site of cell division |  | 16 |
| 4975729571 | chromatin | DNA spread through nucleus like web |  | 17 |
| 4975729572 | chromosomes | DNA becomes rod-shaped as cell begins division; includes 2 long DNA molecules and histone proteins |  | 18 |
| 4975729573 | histones | organize long DNA |  | 19 |
| 4975729574 | nucleosomes | coiling of DNA by histones |  | 20 |
| 4975729575 | nucleoli | concentrations of DNA in process of manufacturing ribosomes |  | 21 |
| 4975729576 | nuclear pores | passageway for proteins and RNA |  | 22 |
| 4975729577 | nuclear envelope | 2 bilipid layers; bounded to nucleus, continuous with ER |  | 23 |
| 4975729578 | ribosome | 2 RNA subunits + proteins; free v bound; protein synthesis |  | 24 |
| 4975729579 | free ribosomes | in cytosol; proteins made function within cell EX: enzymes that catalyze sugar breakdown |  | 25 |
| 4975729580 | bound ribosomes | attached to ER or nuclear envelope; proteins made function within cell membrane or exported from cell |  | 26 |
| 4975729581 | smooth ER | w/o ribosomes; synthesis of lipids and steroids, metabolizes CHO, detoxification |  | 27 |
| 4975729582 | rough ER | w/ ribosomes; synthesis of proteins and glycoproteins, produces new membrane |  | 28 |
| 4975729583 | golgi apparatus | collect, modify, and package proteins, CHO and lipids |  | 29 |
| 4975729584 | lysosomes | (animal cells only) vesicles from Golgi with hydrolytic enzymes; break down material in cytosol for recycling; low pH |  | 30 |
| 4975729585 | peroxisomes | animals: breakdown H202, fatty acids, AAs; plants: modify by-products of photosynthesis |  | 31 |
| 4975729586 | mitochondria | carry out cellular respiration; two membranes allow separation of metabolic processes |  | 32 |
| 4975729587 | chloroplasts | (plant cells only) carry out photosynthesis; two membranes |  | 33 |
| 4975729588 | microtubules | made of protein tubulin; found in spindle apparatus (guides chromosome mvmnt in cell division); support and motility for cell activity |  | 34 |
| 4975729589 | intermediate filaments | support for cell shape |  | 35 |
| 4975729590 | microfilaments | made of protein actin; found in cells that move by shape change, e.g. muscle cells; cell motility |  | 36 |
| 4975729591 | flagella | long, few, snake-like mvmnt; 9+2 microtubule EX: sperm |  | 37 |
| 4975729592 | cilia | short, many, back-and-forth mvmnt; 9+2 microtubule EX: line respiratory tract |  | 38 |
| 4975729593 | centrioles | (animal cells only) microtubule organizing centers; create spine apparatus in cell division |  | 39 |
| 4975729594 | transport vesicles | move materials btwn organelles |  | 40 |
| 4975729595 | food vacuoles | receive nutrients; usually merge with lysosomes |  | 41 |
| 4975729596 | contractile vacuoles | collect and pump water in cell |  | 42 |
| 4975729597 | central vacuoles | (plant cells only) contain most of plant cell interior; exert tugor when full for cell rigidity; functions specialized: 1) store starch, nutrients, waste, etc. 2) lysosome function 3) cell growth by absorbing H20 4) renders large SA-to-V ratio |  | 43 |
| 4975729598 | cell walls | (plant cells only) support |  | 44 |
| 4975729599 | extracellular matrix | (animal cells only) mechanical support, helps bind adjacent cells |  | 45 |
| 4975729606 | prokaryotes | plasma membrane, DNA, ribosomes, cytoplasm, cell wall |  | 46 |
| 4975729607 | hypertonic | solute hypertonic to solution=higher [solutes] |  | 47 |
| 4975729608 | hypotonic | solute hypotonic to solution=lower [solutes] |  | 48 |
| 4975729609 | isotonic | [solute]=[solution] |  | 49 |
| 4975729611 | passive transport | [higher] to [lower]; increases w/ increase in [x], temp., smaller particle size |  | 50 |
| 4975729612 | diffusion | random mvmnt leads to net mvmnt from [high] to [low] |  | 51 |
| 4975729613 | osmosis | diffusion of water across selectively permeable mmbrn |  | 52 |
| 4975729614 | turgor pressure | osmosis into cell |  | 53 |
| 4975729615 | plasmolysis | osmosis out of cell |  | 54 |
| 4975729616 | cell lysis | swelling of cell b/c excess turgor pressure |  | 55 |
| 4975729617 | facilitated diffusion | diffusion through channel or carrier proteins |  | 56 |
| 4975729618 | active transport | [lower] to [higher]; requires use of E (usually ATP) |  | 57 |
| 4975729619 | electrochemical gradient | combo. of concentration and electrical voltage gradients of ions |  | 58 |
| 4975729620 | cotransport | protein that allows downhill mvmt to drive another uphill; E for uphill from [gradient] from downhill |  | 59 |
| 4975729621 | vesicular transport | uses vesicles to move substances across plasma mmbrn |  | 60 |
| 4975729622 | exocytosis | fuse w/ membrane, release contents outside cell |  | 61 |
| 4975729623 | endocytosis | capture substance outside cell, fuse w/ membrane, release contents into cell; 3 types: phagocytosis, pinocytosis, receptor-mediated |  | 62 |
| 4975729624 | phagocytosis | cellular eating; undissolved material enters cell; forms phagocytic vesicle |  | 63 |
| 4975729625 | pinocytosis | cellular drinking; dissolved material enters cell; forms liquid vesicle |  | 64 |
| 4975729626 | receptor-mediated endocytosis | specific molec. (ligands) binds to site, resulting in pinocytosis |  | 65 |
| 4975729627 | water potential | movement of water from where there is high potential to low potential; based upon solute and pressure components | 66 | |
| 4975729628 | solute potential | =-iCRT i = ionization constant (NaCl = 2, glucose = 1) C = concentration (M) R = constant (0.0831 mol-liters/bar K) T = temperature (K) more solute = lower overall potential | 67 | |
| 4975729629 | pressure potential | measurement of pressure, in an open container usually = 0 |  | 68 |



























































































































